What Condition Is Necessary for a Process to Be Adiabatic
This means that the total heat of the. The necessary conditions for adiabatic process are - a There should not be any exchange of heat between the system and its surrounding.

What Is The Work Transfer For Adiabatic Process And Its Derivation Quora
The assumption of no heat transfer is very important since we can use the adiabatic approximation only in very.

. Adiábatos impassable is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environmentUnlike an isothermal process an adiabatic process transfers energy to the surroundings only as work. The system must be completely insulated from its surroundings. For example a reaction that takes place in a Dewar Flask is adiabatic.
The necessary conditions for adiabatic process are-a There should not be any exchange of heat between the system and its surrounding. For a process to be adiabatic the process has to be well insulated. As a key concept in thermodynamics the.
The system can be considered to be perfectly insulatedIn an adiabatic process energy is transferred only as work. Processes that occur very quickly and for which the system does not have time to exchange heat with its surroundings can also be considered to. The word adiabatic means isolated from surroundings.
An adiabatic process is a thermodynamic process in which there is no heat transfer into or out of the system Q 0. The following conditions are necessary for the process to be an adiabatic one. See full answer below.
The assumption of no heat transfer is very important since we can use the adiabatic approximation only in very rapid processes. In thermodynamics an adiabatic process Greek. What is special about an adiabatic process.
Conditions for adiabatic change. Conditions required for an adiabatic process to take place are. In thermodynamics an adiabatic process is defined as a process in which theres no heat exchange in the system.
In an adiabatic process energy is transferred only as work. For an adiabatic process where there is no heat exchanged ΔEW. An adiabatic process is a thermodynamic process in which there is no heat transfer into or out of the system Q 0.
What is the adiabatic form of the first law of thermodynamics. First the system must be properly insulated from the surroundings. C Change of pressure of the gas must be made very rapidly so that there is no chance of heat exchange with the surroundings.
For a process to be adiabatic the process has to be well insulated. B Thermal capacity at the surroundings should be low. The system must.
Any process in which no heat is allowed to be exchanged with surroundings is called an adiabatic process. An isothermal process is a thermodynamic process occurring at a constant temperature. What condition is necessary for a process to be adiabatic.
I System is perfectly insulated from surrounding. A The gas is to be kept in a bad-conducting container. This type of process occurs when the thermodynamic system in this case an ideal gas is enclosed in an adiabatic container with an adiabatic wall.
The condition that the entropy of the system is constant implies that the system is thermally insulated ie that there is no exchange of heat. Ii The process must be carried out rapidly so that the system hs sufficient time to exchange heat with the surroundings. An adiabatic process is one that occurs without transfer of heat or matter between a thermodynamic system and its surroundings.
The thermodynamic process must not get enough time to exchange heat between the system and the surrounding. 2 The adiabatic process provides a rigorous conceptual basis for the theory used to expound the first law of thermodynamics and as such. Fill in the blanks in the following paragraph to correctly identify four principles of the second law of thermodynamics.
For example experiments involving the propagation of sound waves through solids measure the adiabatic compressibility because no heat flow occurs into or out of a small volume of the solid during the time that the pressure. Describe the conditions needed for a process to be isobaric. The adiabatic process is a process that takes place at the constant entropy S Constant dS 0.
1 point Question 3 5 points a. There are two necessary conditions required for an adiabatic process to take place. The following conditions are needed to be fulfilled for adiabatic changes.
For the heat transfer to occur in a sufficient amount of time the process must be performed quickly. The system is perfectly insulated from the surrounding. The system can be considered to be perfectly insulatedIn an adiabatic process energy is transferred only as work.
No heat should enter or leave the system. All walls of the container and the piston must be perfectly insulating. 5 rows Following are the essential conditions for the adiabatic process to take place.
The temperature must be maintained as well. Air temperature rises or falls as pressure increases or decreases. An adiabatic process is a process which takes place without transfer of heat Q 0.
For any physical system in the T-S plane an adiabatic process is represented by a straight line parallel to the T-axis as shown in Fig. The adiabatic compressibility is the fractional change in volume with pressure under constant entropy ie isentropic or adiabatic conditions. All walls of the container and the piston must be perfectly insulating.
Second the process should be very quick such that there is no time energy to be transferred from the system to the environment or from the surroundings to the system. Essential conditions for the adiabatic process to take place are. The temperature must be maintained as well.
Characteristic by parts of air parcels pressure lessens allowed to expand and cool. Correct option is C An adiabatic process is one that occurs without transfer of heat or matter between a thermodynamic system and its surroundings. Adiabatic process means a process that neither allows the heat to transfer inside nor lets the heat out of the system.
In an adiabatic process energy is transferred only as work.

What Condition Is Necessary For A Process To Be Adiabatic Lisbdnet Com
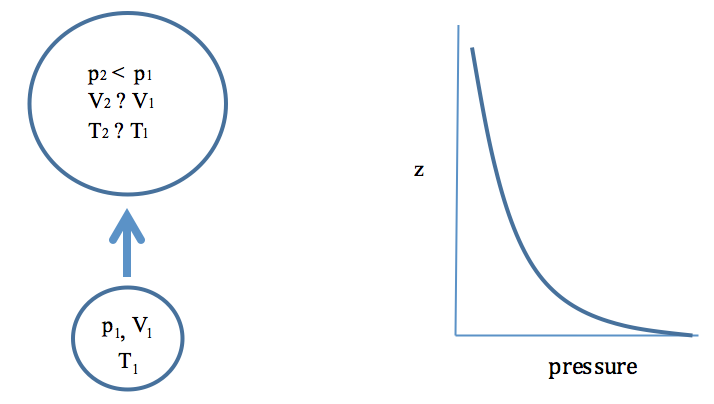
2 5 Adiabatic Processes The Path Of Least Resistance Meteo 300 Fundamentals Of Atmospheric Science

Chapter 3c The First Law Closed Systems Diesel Cycle Engines Updated 3 19 2013

Homework And Exercises Work Done In Isothermal Vs Adiabatic Process Physics Stack Exchange

How To Distinguish Between An Isothermal Adiabatic Process On A Pressure Volume Diagram Physics Study Com
What Is An Adiabatic Process Can An Adiabatic Process Be Done In Real Life If Yes What Are Some Examples Quora
Why Is Sudden Compression Of A Gas An Adiabatic Process Quora

Essential Condition For Adiabatic Process Youtube
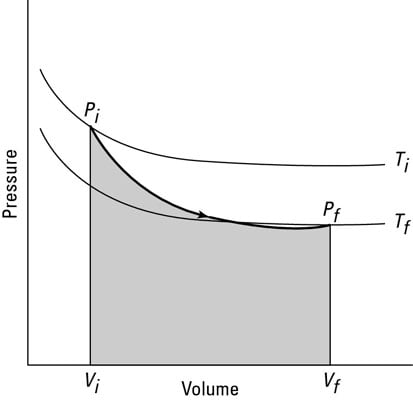
Keeping A System At Constant Heat The Adiabatic Process Dummies

Homework And Exercises Work Done In Isothermal Vs Adiabatic Process Physics Stack Exchange

Adiabatic Process An Overview Sciencedirect Topics
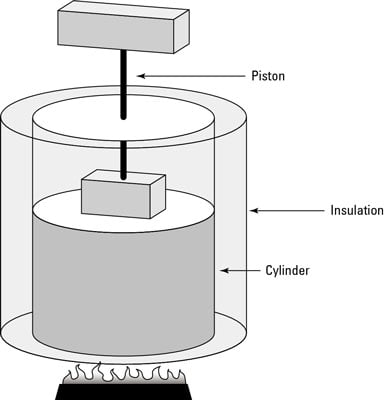
Keeping A System At Constant Heat The Adiabatic Process Dummies
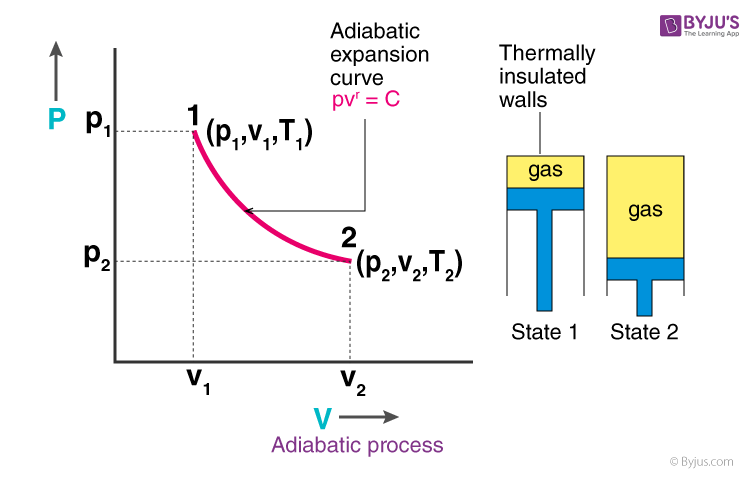
Adiabatic Process Definition Equation Reversible Adiabatic Process Example Differences Video And Faqs
In Layman S Terms What Is The Difference Between An Adiabatic And Isothermal Process Quora

Derivation Of Expression For Work Done In Adiabatic Process Youtube

Adiabatic Processes Physics And Mathematics Lapse Rate Cloud Type

Are All Isoentropic Processes Adiabatic Quora

Comments
Post a Comment